CITRAL
Properties
Citral
is a acyclic monoterpenoid having molecular formula of C10H16O;
Citral is chemically known is 3,7-dimethyl-2,6-octadienal, trivially called as
lemoral; Citral is a clear yellow colour liquid; Melting point of Citral is :
-10 0C; boiling point of Citral is: 770C; Citral is insoluble
in water; Density of Citral is 0.9 g/cm3; Citral is less denser than
water; Citral is optically inactive; Principle source of Citral is lemon grass
oil, other sources includes lemon myrtle, lemon balm, lemon tea tree; Citral is
isolated as its crystalline bisulphite product, which on hydrolysis gives
Citral ; Citral has been used in synthesis of β-ionone and methyl ionone;
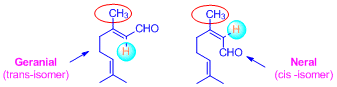
Citral exists in two isomeric forms: [1] Geranial (trans-isomer, it is also
known as Citral-a) , [2] Neral (cis -isomer, it is also known as Citral-b);Citral
contains two isoprene units connected by head to tail fashion;
Structural elucidation
1) The molecular formula of
Citral is C10H16O, which was confirmed by C, H, N
elemental analysis.
2) Citral adds two molecules
of bromine, and catalytic reduction of Citral forms tetrahydro derivatives.
This confirms the presence of 2 double bond in Citral.
3) Citral reduces Fehling’s
solution, this indicates the presence of aldehyde group; moreover, Citral on
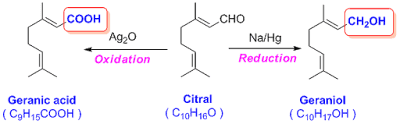
reduction by sodium amalgam forms an alcohol namely Geraniol (C10H18O);
Citral on oxidation by silver oxide forms an acid namely Geranic acid (C10H16O2);
during these two reaction, there is no loss of any carbon atom. This means that
oxygen present in Citral is aldehyde oxygen.
4) The presence of 2 double
bond and one aldehyde group in Citral implies that its correspondent
hydrocarbon must have molecular formula of C10H22 ie.)
the formula for the saturated compound obtained on complete reduction of
Citral. This formula is in the form of CnH2n+2. This is
the general formula for acyclic compound, therefore Citral is acyclic compounds.
5) Citral on heating with
potassium hydrogen sulphate gives well
known aromatic compound
known s p-cymene, whose structure is well established. Thus the carbon skeleton and position of
alkyl (methyl,
isopropyl) groups are
established.
6) Oxidation of Citral with
alkaline KMnO4 followed by Chromic acid gives acetone, oxalic acid
and leavulic acid. This clearly accounts for proposed structure.
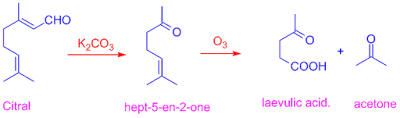
7) The above structure is
also confirmed by the observation that Citral on treatment with K2CO3
gives an aldehyde and an unsaturated ketone. The ketone was identified as
hept-5-en-2-one. This ketone on treatment with ozone gives acetone and laevulic
acid.